One molecule fits all
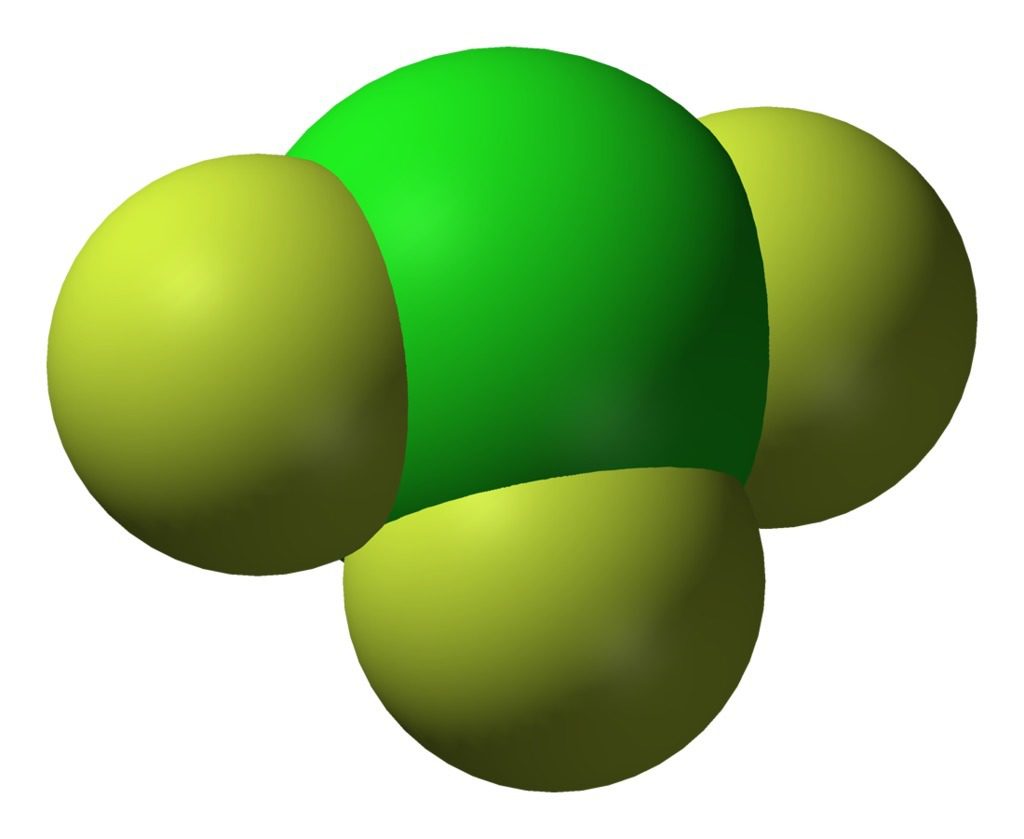
Common in school laboratories world-wide, hydrochloric (or muriatic) acid (chemical formula HCl) is a very simple molecule: it is simply one atom of hydrogen and one atom of chlorine that have exchanged one electron, thus forming an electrically charged bond. At room temperature the molecule is a gas, but once dissolved in water it is a powerful and useful reactant for many chemical applications.
On an industrial scale, HCl is used for the purification of table salt, leather processing, removing sulfur from petrol, hydraulic fracturing, gelatin production, in sanitation and for the synthesis of vinyl chloride and its derived plastic PVC (polyvinyl chloride). Hydrochloric acid is also used on a large scale for “pickling” metals: this is a chemical cleaning technique that removes impurities, stains, rust or scale from the surface of ferrous metals, copper and aluminium alloys.
Indirectly, it is used in brewing and sugar refining, in electronic silicone, producing metal chlorides for fireworks, in making batteries, pharmaceuticals, rocket propellants, computer chips, mobile phones, unmanned aerial vehicles (UAVs) and many more!
It is even found in our bodies. Read more on hydrochloric acid in your stomach here.
If you saw a bottle of hydrochloric acid in a chemical laboratory, you would say it looks exactly like water. But it is much more aggressive, highly corrosive and very pungent. It’s strong stuff that acid... to be handled with much care!