How are chlorine and caustic soda made?
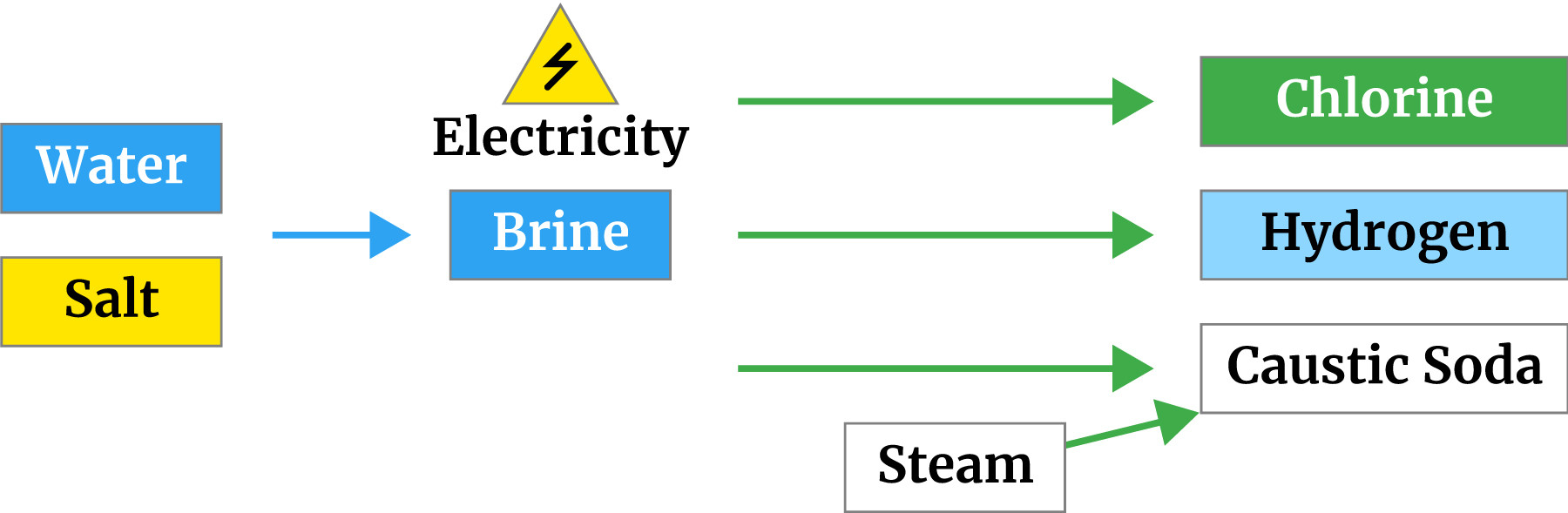
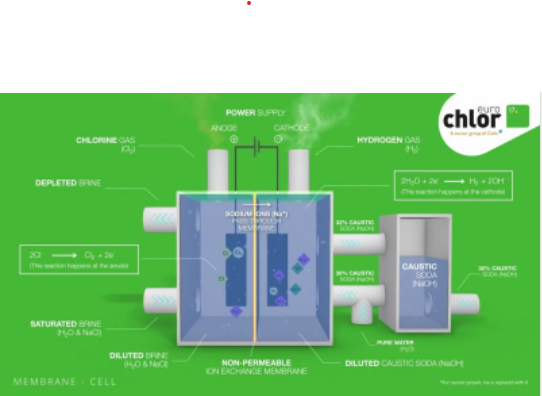
Chlorine occurs naturally but not in its elemental (gas) form (as Cl2). Caustic soda (usually as NaOH) is produced as a liquid. These are produced by passing an electrical current through brine (common salt dissolved in water). This is called electrolysis, a process which has been known for over 120 years.
There are three key ingredients to make elemental chlorine; salt, electricity and water. From these three ingredients, we get elemental chlorine (Cl2), caustic (often sodium hydroxide or NaOH) and hydrogen (H2). As these three products are highly reactive, technologies have been developed to keep them apart.
Chlorine production plant by night – Courtesy of Vynova Group
The three main technologies for producing chlorine are:
- the membrane cell process, most widely used in Europe (85% of installed capacity),
- the mercury cell process, now phased out in Europe and
- the diaphragm cell process (used for nearly 10% of installed Europeancapacity)
Other technologies for producing chlor-alkali are also used and these account for around 5% of installed capacity.
A typical membrane chlorine production cell room – Courtesy of Vynova Group