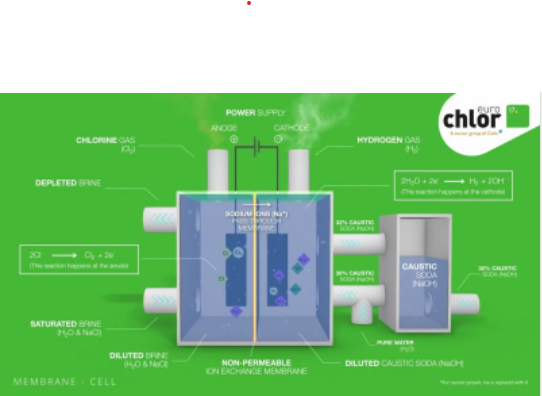
Membrane cell process
Two electric connection points of each chlorine production cell, the anode and the cathode, are separated by an ion-exchange membrane. This membrane allows only sodium ions and a small amount of water to pass through it as they move towards the negatively charged cathode.
On the cathode, hydrogen gas forms as water is split, which bubbles out and is collected. The caustic solution that remains leaves the cell at about 30% concentration before often being further concentrated to 50% away from the cell.
On the other side of the membrane, chlorine bubbles out at the anode meaning that the ‘spent’ brine can be resaturated with more solid salt before it is purified using an ion exchanger. The chlorine gas contains some oxygen and must often be purified by liquefaction and evaporation.
The consumption of electric energy is the lowest of the three processes and the amount of steam needed to concentrate the caustic is relatively low (less than one tonne per tonne of caustic soda).